<a href="http://www.wordle.net/show/wrdl/2530510/proton"
title="Wordle: proton"><img
src="http://www.wordle.net/thumb/wrdl/2530510/proton"The Proton was discovered in 1918 by Ernest Rutherford when he sent alpha particles through nitrogen gas (7). It is not always clear who discovered the proton because the process of the discovery was slow and gradual, and many scientists contributed. Thomson and Chadwick helped, but Rutherford is credited with the discovery because he was the first to clearly prove the existence of protons (8).
Ernest Rutherford
- originally from New Zealand, but moved to the UK to become a professor and teach future scientists (8)
- won the Nobel Prize, was honored with knighthood, and overall became an icon (8)
- because of the discovery of the proton he is referenced to as the "father of nuclear physics"(9)
What is a proton?
- a nucleon (9) that produces a positive electrical charge (1)
- composed of two "up" quarks and one "down" quark (1)
- a quark is an elementary particle (2)
- gluons hold quarks together (4)
- a subatomic particle found in the nucleus of an atom (7)
- a proton can be bounded by nuclear force and converted to atomic nuclei (9)
- a proton without neutrons is usually a nucleus of a common hydrogen atom isotope (9)
The Experiment that proved the proton's existence:
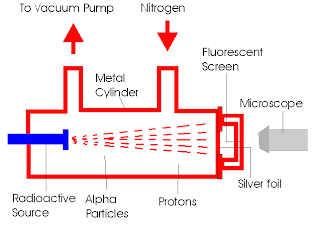
- Rutherford discovered the proton when he noticed the marks of hydrogen nuclei being released as the process of nitrogen was being converted into oxygen through a nuclear reaction involving the shooting of alpha particles into nitrogen gas (10)
- Rutherford observed after shooting alpha particles into nitrogen gas that the scintillation (sparkle of light) detectors showed hydrogen nuclei indications that hydrogen could only have come from nitrogen (9)
- Rutherford concluded nitrogen must be comprised of hydrogen nuclei
- He named the hydrogen nucleus, with atomic number one, proton (9), which is "first" in Greek (3)
 |
| equation that explains nuclear reaction(10) |
 |
| nuclear transmutation taking place (turning an element into another)(10) |
- Rutherford performed another experiment where he shot alpha particles(helium nuclei produced by radioactive decay) at gold foil because he was trying to prove the model of an atom (4)
- unpredicted from what he thought, some of the alpha particles passed through the gold foil, and others bounced back (4)
- Rutherford recognized from his experiment that the alpha particles bounced back because the atom was made of a dense nucleus surrounded by a cloud of orbiting electrons (4)
- once the electron was discovered, Rutherford reasoned that there were positive charge centers within the atom to balance the negative electrons and create electrically neutral atoms (6)
- Rutherford's discovery of the nucleus proves that the positive charges were concentrated in a very small fraction of the atom's volume (6)
- Rutherford and other physicists compared the nuclear masses to charges and realized that the positive charge of any nucleus could be accounted for by an integer number of hydrogen nuclei (6)
- Video for clearer explanation of the gold foil experiment(proved protons were positive charged particles) below
(11)
Importance that came from the discovery of the proton:
- milestone in the development of atomic theory because it provided a greater understanding of how molecules bond and work (7)
- Rutherford discovered he could change one element into another by striking it with energetic alpha particles, helium nuclei, where in each case the helium nuclei were released in the process (6)
- number of protons in the nucleus of an atom determines which chemical element the atom is (7)
- scientists believes a proton lives for at least 10^31 years (2)
- the Large Hadron Collider(LHC) is the world's most powerful particle accelerator and can smash protons (5)
- In 1920 protons first appeared in print(newspapers, magazines) (6)
Work Cited
1. http://www.physicsoftheuniverse.com/scientists_rutherford.html
2."Proton." Columbia Electronic Encyclopedia, 6th Edition (2009): 1. Vocational and Career Collection. EBSCO. Web. 30 Sept. 2010.
3."Chadwick Proves the Existence of the Neutron." 379. Salem Press/Magill Books, 1999. Science Reference Center. EBSCO. Web. 30 Sept. 2010.
4."Holey grail." Economist 367.8319 (2003): 72. MAS Ultra - School Edition. EBSCO. Web. 30 Sept. 2010.
5. Castelvecchi, Davide. "Bring it on!." New Scientist 193.2588 (2007): 36. Science Reference Center. EBSCO. Web. 30 Sept. 2010.
6.http://www.physlink.com/education/askexperts/ae46.cfm
7.http://www.ehow.com/facts_5183474_discovery-proton-neutron.html
8. http://www.articledashboard.com/Article/The-Story-of-The-Proton-Discovery-and-the-People-Behind-it/1580858
9. http://www.everydayguide.com/who-discovered-the-proton/
10. http://library.thinkquest.org/27954/proton.html
11. http://www.youtube.com/watch?v=bSEOOMs5VNU
11. http://www.youtube.com/watch?v=bSEOOMs5VNU
Wowzers! haha Macry your blog was very impressive! i was having a lot of trouble trying to come up with something you could do better. When i came across words i didn't know i liked how you had a brief definition underneath the word (that was a very helpful tool) Each picture and movie was also good to help the reader further understand the experiments Rutherford did. The only thing i can suggest to you is maybe under each picture along with caption write in your own words whats happening in the picture. Also maybe next time describe why certain reactions occurred.
ReplyDeleteGreat job Marcy!.This blog is awesome. I really like the way you organized all of your information. This must have taken a lot of time! The pictures are so discriptive and go along with the information so well. My favorite part is the fun facts. How interesting! I can't find anyway you could improve this blog, so perfect!
ReplyDeleteGreat job on your blog! It was very well organized. I liked how you included the fun facts. Some of your descriptions are a bit unclear, you could have gone more in-depth about how he made some his conclusions. How did his gold foil experiment really lead to his conclusion, i.e. how did he come to decide that the nucleus was made up of positive charges. I liked how you added lots of pictures, they corresponded with your explanations very well.
ReplyDeleteMarcy, you did a wonderful job in every single aspect. It flowed well, the pictures were very helpful and informative, and the YouTube video was the icing on the cake in terms of the thoroughness of your blog. You have a lot of specific information without getting too technical. The fun facts was a nice touch as well. You did a superb job and I enjoyed reading your blog!!
ReplyDeleteGreat job on your blog Marcy! It was very informative without being over the top, and the way you organized your blog helped to clearly explain discovery of the proton. The pictures and videos were great visuals that contributed to the explanation as well. I thought you found some great information that was very informative. Great job!!
ReplyDelete- Christina B.
Prof Prem raj Pushpakaran writes -- 2019 marks the 100th year of the discovery of positively charged stable subatomic particle, Proton!!!
ReplyDelete